Scientists Grow “Synthetic” Mouse Embryo – With Brain and Beating Heart – From Stem Cells
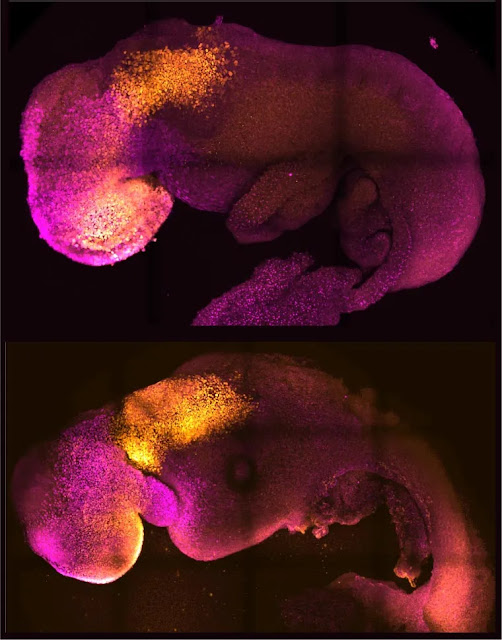
The folds of the heart and the head are colored, and there are natural and artificial embryos side by side. By permission of M. Zernicka-Goetz
From stem cells, scientists have developed model mouse embryos with functioning hearts, brains, and all of the other organs found in a mouse's body. The body's master cells, stem cells have the ability to differentiate into practically any type of cell. Researchers from the California Institute of Technology and the University of Cambridge carried out the research (Caltech).
The findings, which represent the culmination of more than ten years of research, may shed light on why some embryos fail to develop into fetuses while others do so as part of a successful pregnancy. The outcomes could also be utilized to direct the production and repair of artificial human organs for transplantation.
The discovery is detailed in a report published in the journal Nature today (August 25). Magdalena Zernicka-Goetz, a Bren Professor of Biology and Biological Engineering at Caltech, oversees the lab where the study was carried out. In Cambridge's Department of Physiology, Development, and Neuroscience, Zernicka-Goetz also holds the positions of professor of mammalian development and stem cell biology.
The embryo model was created without the usage of sperm or eggs. Instead, the scientists were able to replicate natural processes in the lab by directing the three different types of stem cells that are present in early mammalian development to the point where they start interacting. By increasing the expression of a certain set of genes and creating a special environment for their interactions, the researchers were able to induce the stem cells to "speak" to one another.
Comparing the development of the brain and heart in natural and synthetic embryos, they are similar. Credit: Handford and Amadei
The synthetic embryos eventually had beating hearts and the building blocks for brains because the stem cells gradually self-organized into structures that advanced through the various developmental stages. Even the yolk sac, where the embryo grows and obtains nutrition during its early weeks, was present. This is the stage of development that a stem cell-derived model has reached the most recently.
The capacity to produce the full brain, in particular the anterior area, which has been a "holy grail" in the production of synthetic embryos, is a significant advancement in this research.
According to Zernicka-Goetz, "this gives new opportunities to research the mechanics of neurodevelopment in an experimental model." In reality, by disrupting a gene already known to be crucial for the development of the neural tube, a forerunner to the nervous system, as well as the brain and eyes, we show the confirmation of this notion in the work. The synthetic embryos exhibit the same recognized problems in brain development as an animal with this mutation when this gene is absent. As a result, we can start using this method on the numerous genes whose role in brain development is unknown.
She continues, "Our mouse embryo model develops not only a brain but also a beating heart, all the components that go on to build up the body. The fact that we've come this far is really incredible. This has been our community's long-term goal and the primary focus of our effort for ten years, and we've finally achieved it.
A "conversation" between the tissues that will form the embryo and the tissues that will link the embryo to the mother is necessary for a human embryo to successfully develop. Three different stem cell types begin to form within the first week of fertilization; one of these will eventually develop into the bodily tissues, while the other two will help the embryo's growth. The placenta, which connects the fetus to the mother and supplies oxygen and nutrition, will be formed from one of these latter two types, which are referred to as extraembryonic stem cells. The other will develop into the yolk sac, which is where the embryo develops and gets its early nutrition.
When the three different stem cell types start communicating with one another mechanically and chemically, instructing the embryo how to develop appropriately, many pregnancies end prematurely.
According to Zernicka-Goetz, "this early phase sets the foundation for everything else that follows throughout pregnancy." If something goes wrong, the pregnancy won't succeed.
In order to understand why some pregnancies end in failure and others in success, Zernicka-team Goetz's has been studying these early phases of pregnancy for the past ten years.
The tiny embryo's implantation into the mother's womb ordinarily prevents us from seeing the forming structure at this level, according to Zernicka-Goetz. However, the stem cell embryo model allows us access to the developing structure at this point. This accessibility enables us to modify genes in a model experimental setting to comprehend their developmental roles.
The researchers used cultivated stem cells that represented each of the three tissue types to direct the growth of their synthetic embryo. They allowed them to grow in proportions and a setting that facilitated their interaction with one another and ultimate self-assembly into an embryo.
The scientists found that the extraembryonic cells guide the growth of the embryo by sending chemical signals to the embryonic cells as well as mechanically, or through touch.
"This time of human existence is so enigmatic, so to be able to watch how it happens in a dish—to have access to these specific stem cells, to understand why so many pregnancies fail and how we might be able to prevent that from happening—is pretty extraordinary," says Zernicka-Goetz. We examined the conversation that needs to take place between the various stem cell types at that time—we demonstrated how it happens and how it might go awry.
While the current study used mouse models, researchers are working on a human embryo development similar model to better understand the mechanisms underlying critical processes that would otherwise be impossible to examine in real embryos.
The development of synthetic organs for patients awaiting transplants could be influenced by these techniques if they are later proven effective with human stem cells. According to Zernicka-Goetz, a great number of people worldwide endure lengthy organ transplant waiting lists. The possibility of using the knowledge gained from our research to develop accurate synthetic human organs to save lives that are currently being lost is what makes our work so thrilling. With our current understanding of how adult organs are created, it should also be able to modify and cure them.
"Synthetic embryos complete gastrulation to neurulation and organogenesis" was published in Nature on August 25, 2022.
The study is titled "Anterior brain regions and a beating heart develop in stem cell-derived mouse embryos within an extra-embryonic yolk sac." Gianluca Amadei and Charlotte Handford, both from the University of Cambridge, are the joint first authors. Postdoctoral researchers Hannah Greenfeld and Dong-Yuan Chen, graduate student Martin Tran, professor of biology and bioengineering Michael Elowitz, a Howard Hughes Medical Institute investigator, and research professor of biology and biological engineering David Glover are all co-authors from Caltech. Other authors include Beth Martin and Alejandro Aguilera-Castrejon of the Weizmann Institute of Science in Israel, Chengxiang Qiu and Joachim De Jonghe of the University of Washington, Joachim De Jonghe and Florian Hollfelder of the University of Cambridge, Jay Shendure of the University of Washington, the Brotman Baty Institute for Precision Medicine in Seattle, the Allen Discovery Center for Cell Lineage Tracing in Seattle, and the Howard Hughes Medical Institute
The Wellcome Trust, Open Philanthropy/Silicon Valley Community Foundation, Weston Havens Foundation, the National Institutes of Health, and the Centre for Trophoblast Research all contributed funding to the study.
By CALIFORNIA INSTITUTE OF TECHNOLOGY



Comments
Post a Comment